Description
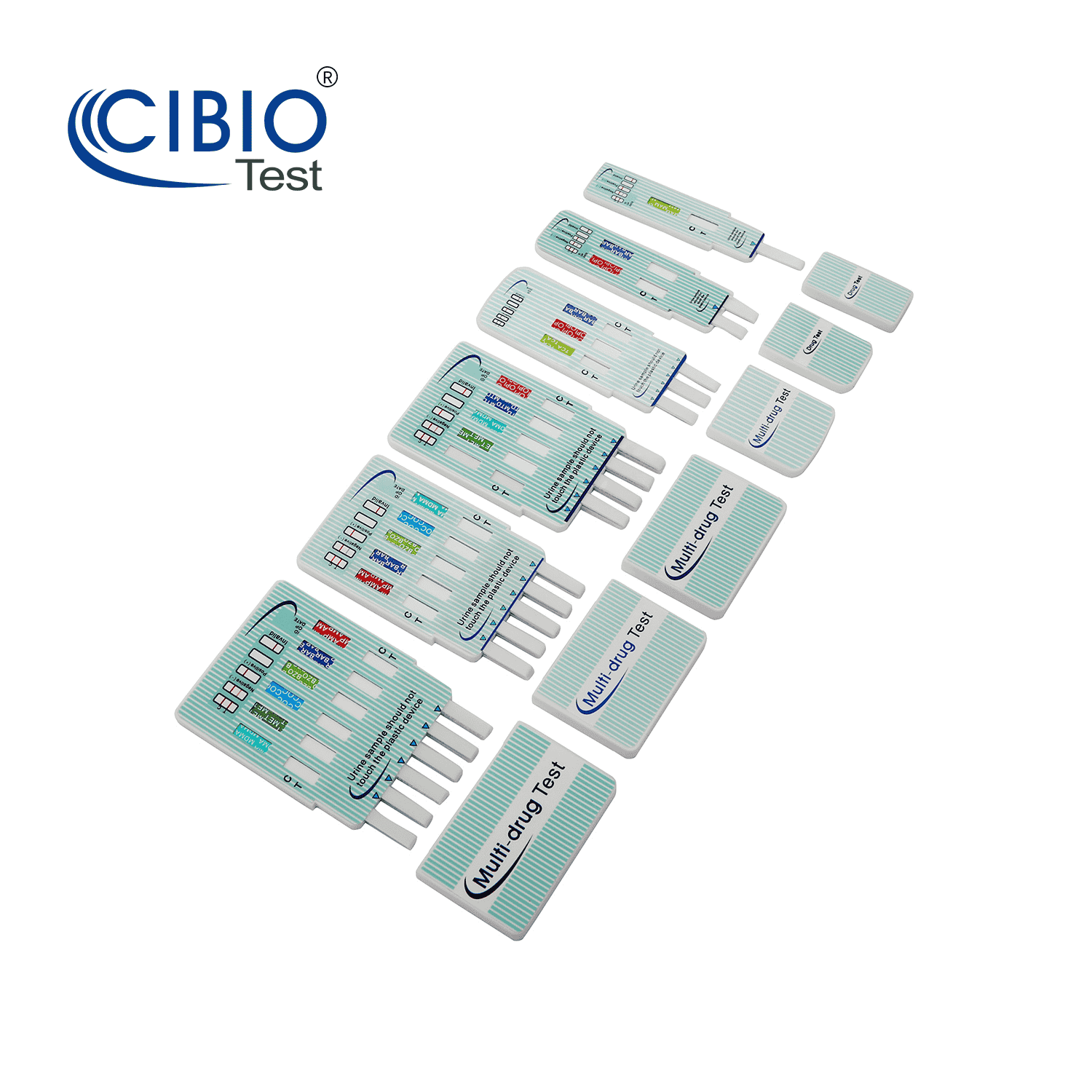
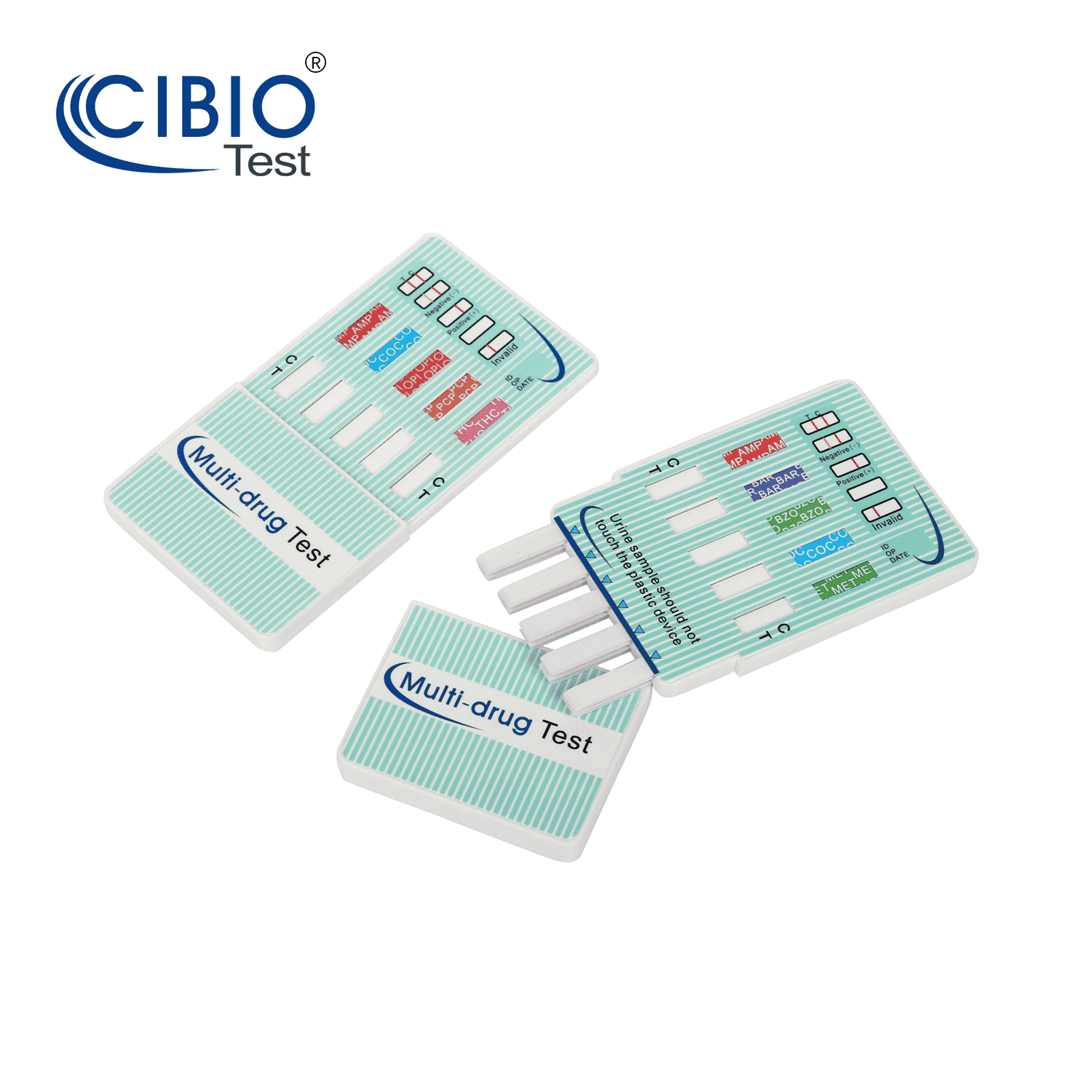
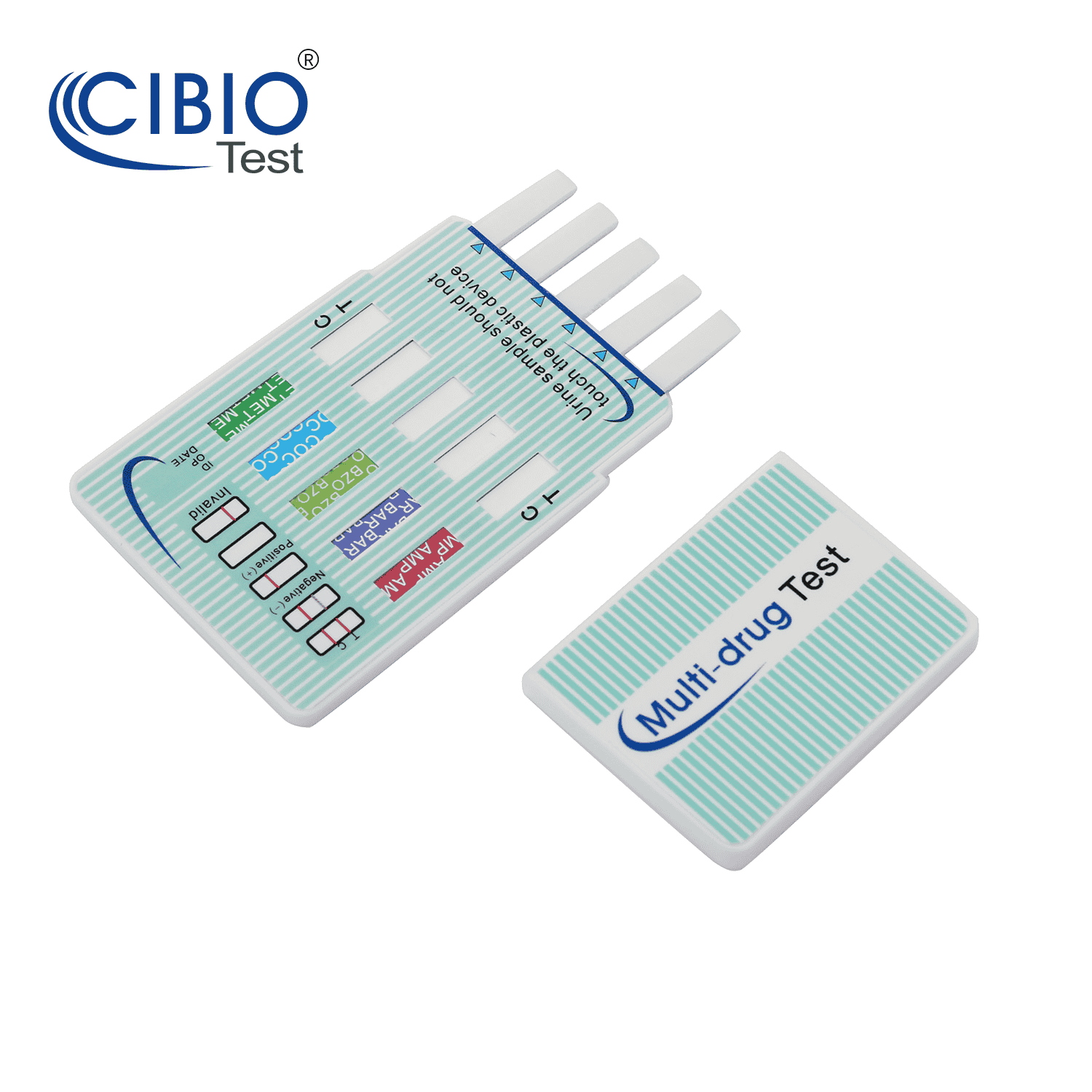
Multi-Drug Test Dip Card
Rapid Multi-drug Test Dipcard is a rapid, screening test for the qualitative detection of multiple drugs and drug metabolites in human urine at specified cut off levels.
For professional use only. For in vitro diagnostic use only.
| Product Name | Multi-Drug Test Dip Card |
| Brand Name | CIBIO® or OEM |
| Format | Multi-drug Test Dip Card |
| Methodology | Colloidal Gold |
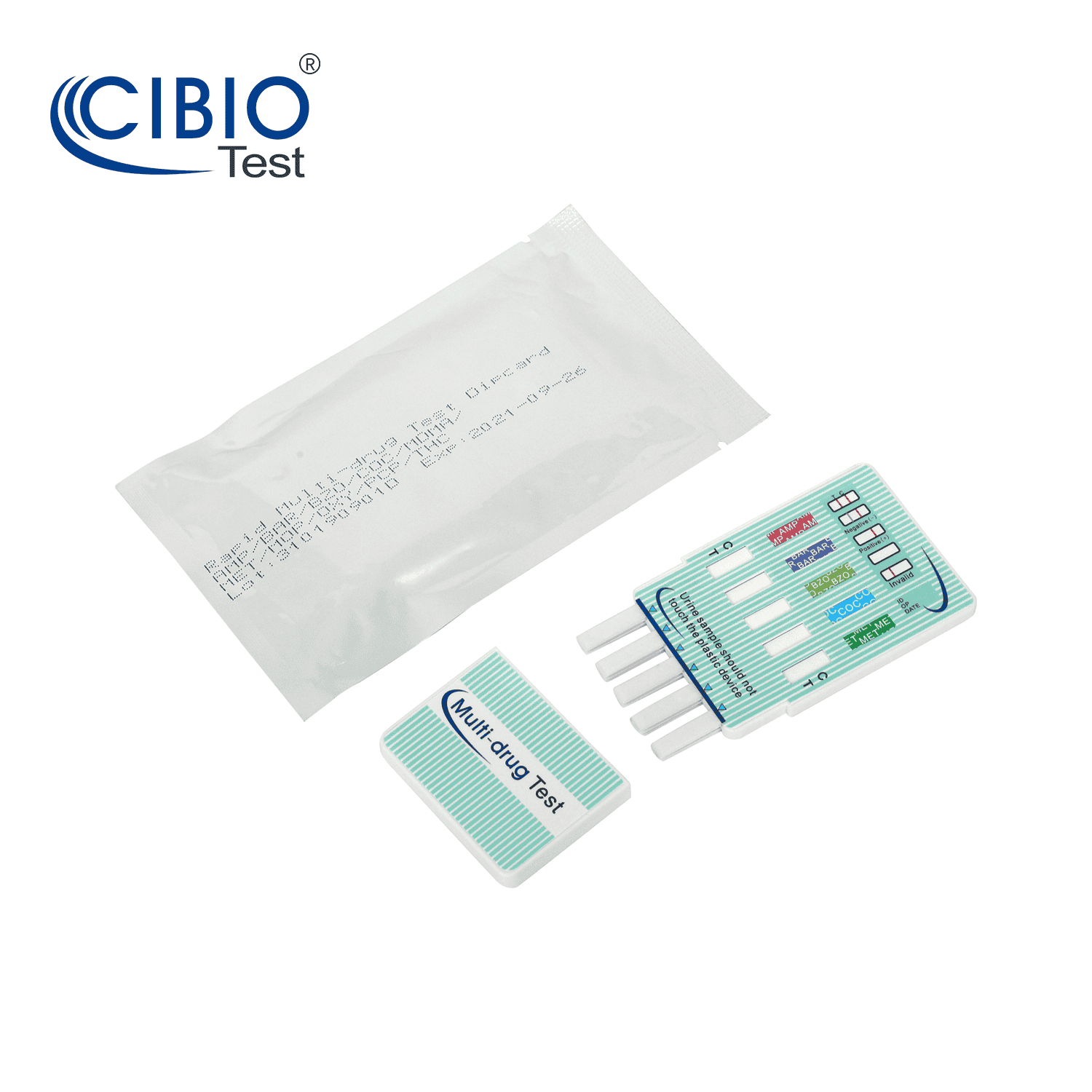
| Kit Contents | 1. Dip Card |
| 2. Desiccant | |
| 3. Instruction for use | |
| Assay Type | Qualitative Detection |
| Specimen | Urine |
| Panels Content | 2-12 panel drug test for Dip Card |
| Certificates | ISO 13485,FDA 510K, Clia Waived |
| Accuracy | Over 99% |
| Storage Temperature | 4-30 degree centigrade |
| Shelf Life | 2 years (24 months) |
| Delivery time | 15-30 days |
| OEM Services | Pouch, Box, Private Label, Insert |
| Loading Port | Guangzhou or Shenzhen, China |
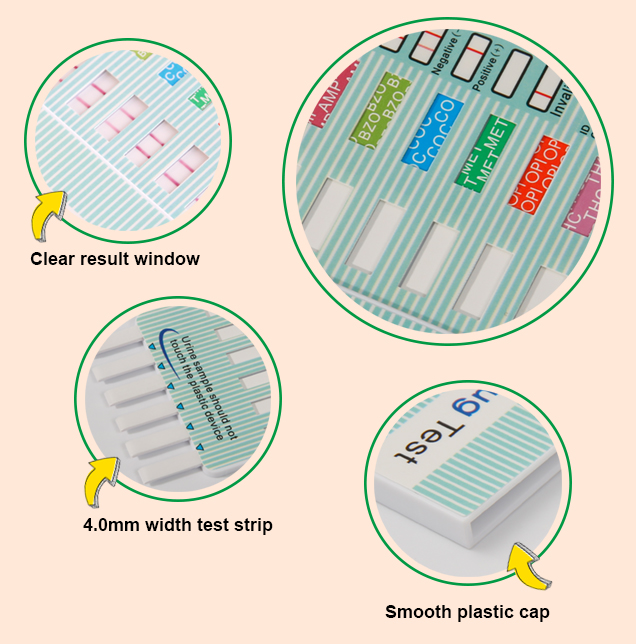
Toxicology urine test list
| Product Description | Abbreviations | Cut-off Level | Qualification | ||
| 1 | Amphetamine | AMP | 1000 ng/mL | CE | FDA 510K,CLIA-waived |
| 2 | Barbiturates | BAR | 300 ng/mL | CE | FDA 510K,CLIA-waived |
| 3 | Buprenorphine | BUP | 10 ng/mL | CE | FDA 510K,CLIA-waived |
| 4 | Benzodiazepines | BZO | 300 ng/mL | CE | FDA 510K,CLIA-waived |
| 5 | Cocaine | COC | 300 ng/mL | CE | FDA 510K,CLIA-waived |
| 6 | Cotinine | COT | 200 ng/mL | CE | |
| 7 | Ethylglucuronide | ETG | 500 ng/mL | CE | |
| 8 | Fentanyl | FYL | 200 ng/mL | CE | |
| 9 | Fentanyl | FYL | 1 ng/mL | FDA 510K | |
| 10 | Synthetic Cannabinoid | K2 | 50 ng/mL | CE | |
| 11 | Ketamine | KET | 1000 ng/mL | CE | |
| 12 | 6-Acetylmorphine | 6-MAM | 10 ng/mL | CE | |
| 13 | Methylenedioxymethamphetamine – ecstasy | MDMA | 500 ng/mL | CE | FDA 510K,CLIA-waived |
| 14 | Methamphetamine | MET | 1000 ng/mL | CE | FDA 510K,CLIA-waived |
| 15 | Morphine | MOP | 300 / 2000 ng/mL | CE | FDA 510K,CLIA-waived |
| 16 | Methadone | MTD | 300 ng/mL | CE | FDA 510K,CLIA-waived |
| 17 | Opiate 2000 | OPI | 2000 ng/mL | CE | |
| 18 | Oxycodone | OXY | 100 ng/mL | CE | FDA 510K,CLIA-waived |
| 19 | Phencyclidine | PCP | 25 ng/mL | CE | FDA 510K,CLIA-waived |
| 20 | Propoxyphene | PPX | 300 ng/mL | CE | FDA 510K,CLIA-waived |
| 21 | Tri-cyclic Antidepressants | TCA | 1000 ng/mL | CE | FDA 510K,CLIA-waived |
| 22 | Marijuana | THC | 50 ng/mL | CE | FDA 510K,CLIA-waived |
| 23 | Marijuana | THC | 20 / 50ng/mL | FDA 510K,CLIA-waived | |
| 24 | Tramadol | TRA | 200 ng/mL | CE | |
| 25 | Xylazine | XYL | 1000 ng/mL | ||
| 26 | Methaqualone | MQL | 300 ng/mL | CE | |
| 27 | Carisoprodol | SOMA | 1000 ng/mL | ||
| 28 | Methcathinone | MCAT | 500 ng/mL | ||
| 29 | Lysergic Acid Diethylamide | LSD | 20 ng/mL | ||
| 30 | Kratom | KRA | 500 ng/mL | ||
| 31 | 3,4-Methylenedioxypyrovalerone | MDPV | 1000 ng/mL | ||
| 32 | Tianeptine | TIA | 500 ng/mL | ||
| 33 | Gabapentin | GAB | 1000 ng/mL | ||
Optional Adulterant Strips: CR – Creatinine, OX – Oxidants, pH – Acidic or Alkaline, S.G – Specific Gravity, NIT – Nitrite, GLU – Glutaraldehyde
How to do the test?
Test must be in room temperature 59-86°F (15-30°C).
1. Open the sealed pouch by tearing along the notch. Remove the test dipcard from the pouch.
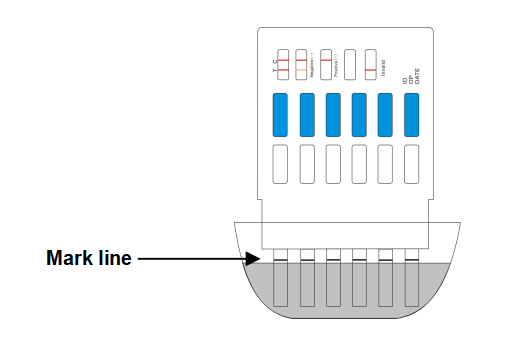
2. Immerse the dipcard into the urine with the arrow pointing towards the urine. Take the dipcard out after 10 seconds.
IMPORTANT: Do not allow the urine level to exceed the MAX (marker line), otherwise the test will not perform correctly.
3. Lay the test dipcard on a clean, dry, non-absorbent surface.
4. Read the results at 5 minutes. The drug test results remain stable for up to thirty minutes.
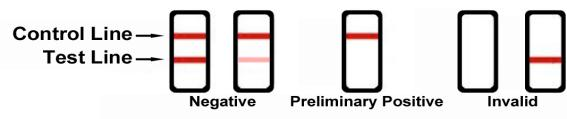
Reading the results
Preliminary positive (+)
A rose-pink band is visible in each control region. If no color band appears in the appropriate test “T”region, a preliminary positive result is indicated for the corresponding drug of that specific test zone.
Negative (-)
If a rose-pink band is visible in each control region and the appropriate test “T” region, it indicates that the concentration of the corresponding drug of that specific test zone is absent or below the detection limit of the test.
Invalid
If a color band is not visible in the control “C” region or a color band is only visible in the test “T” region, the test is invalid. Another test should opened and run to re-evaluate the specimen. If test still provides an invalid result, please contact the distributor from whom you purchased the product. When calling, be sure to provide the lot number for the test.
Q&A
Qus: How do rapid drug tests work?
Rapid drug tests detect the presence of drugs and drug metabolites in human urine at specified cut off levels in minutes. When a detection strip is wetted with urine specimen, the specimen reacts with the reagents and antigens on the strip and produces a color reading so you can interpret a positive or negative result.
Qus: What is the difference between a Dip Card Test and an Integrated Cup Test?
The Dip Card is dipped into a urine specimen to get results. A cup is a convenient way that the specimen is collected in the cup and tested there as well. There is no handling of the specimen.
Qus: What is the shelf life of your drug testing products?
The rapid drug test products have a shelf life of 24 months from manufacturing date. The expiration date is on every individual pack.
Qus: The drug line is lighter than the control line. Does this mean some drug is present?
No. Any line next to the word Drug or the drug abbreviation (depending on the test you have purchased), no matter how dark or light, is considered a Negative Result and no further testing is required.
Qus: Why is Urine Testing the Most Frequently Used Method for Drug Testing?
Businesses choose to drug test their workers and new job applicants to reduce the risk of workplace drug use and relevant hazards. While all drug test types, like hair follicle, blood, and saliva, have their benefits, urine drug testing is the most flexible and customizable.



-300x300.jpg)
Reviews
There are no reviews yet.