Description
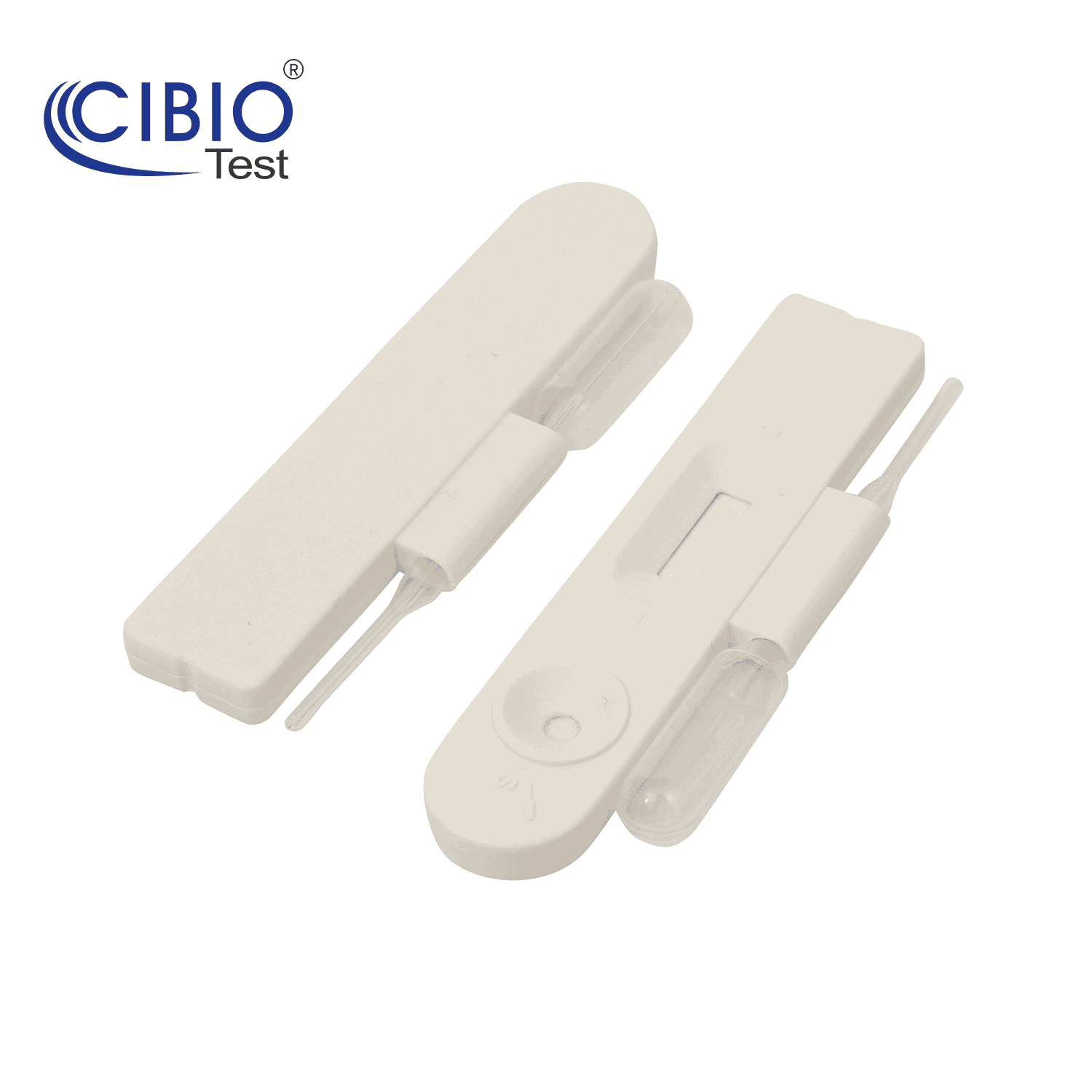
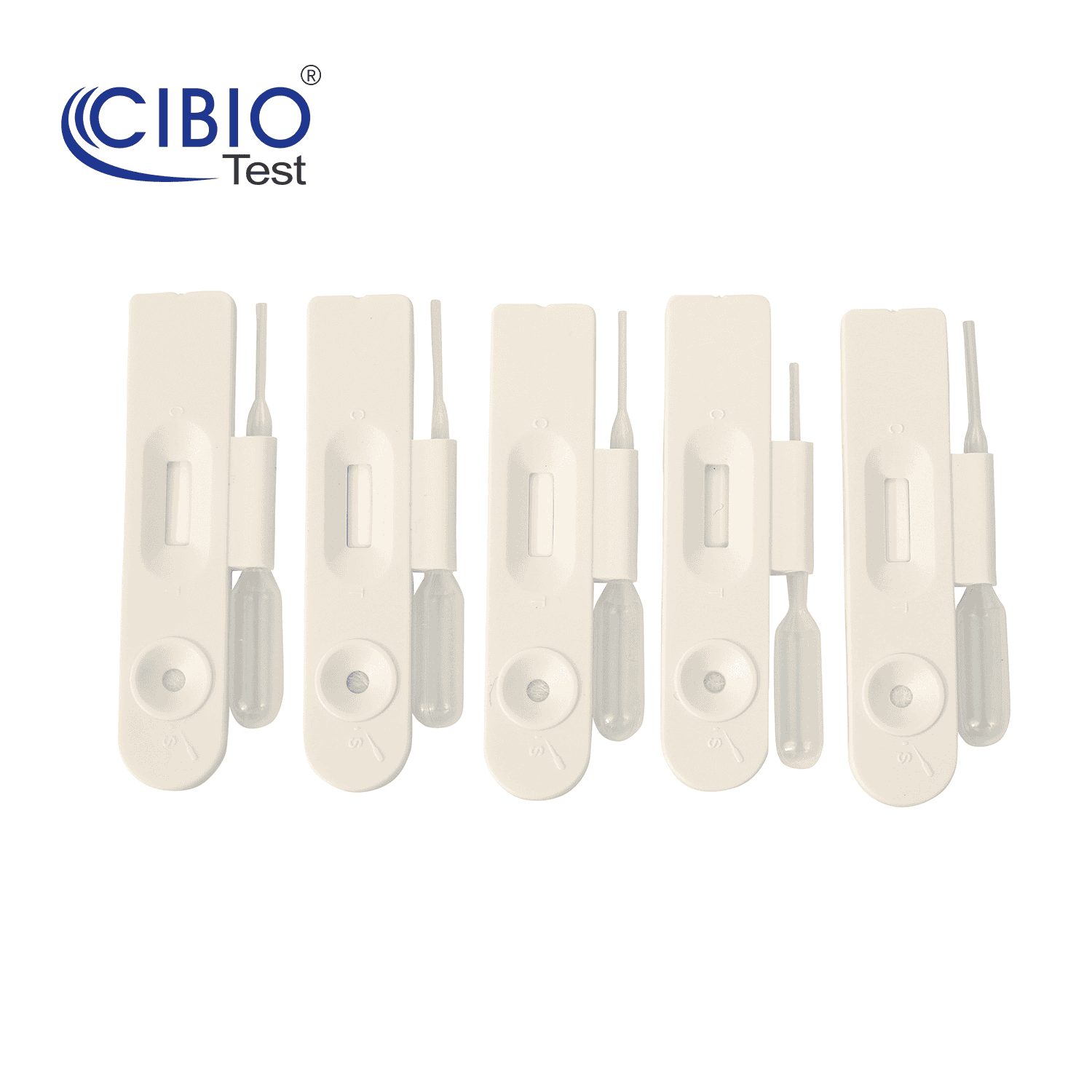
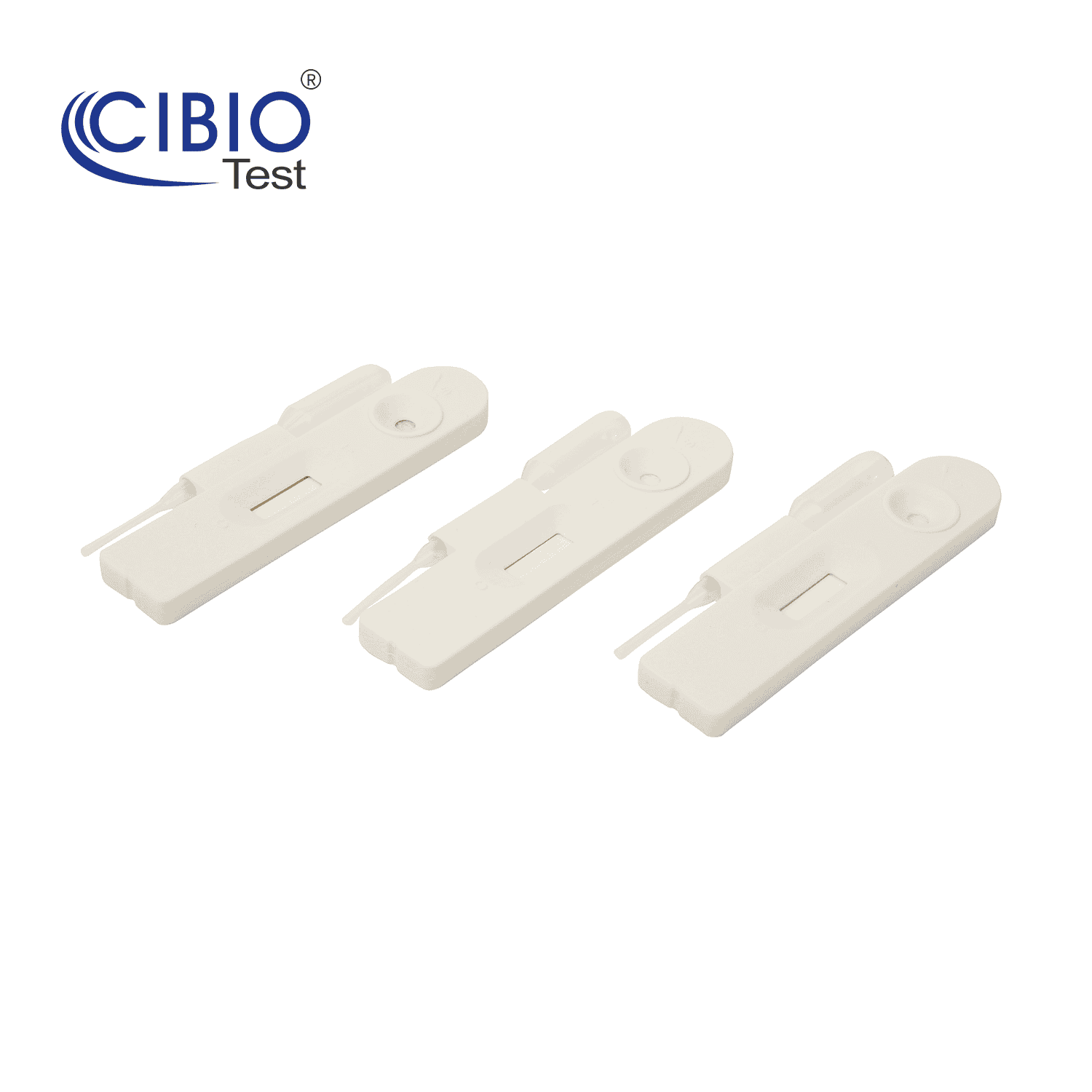
Single Panel Drug Test Cassette
Rapid Single Drug Test Cassette is a rapid, screening test for the qualitative detection of single drug and drug metabolites in human urine at specified cut off levels.
For professional use only. For in vitro diagnostic use only.
| Product Name | Single Panel Drug Test Cassette |
| Brand Name | CIBIO® or OEM |
| Format | Cassette |
| Methodology | Colloidal Gold |
| Kit Contents | 1. Cassette |
| 2. Desiccant | |
| 3. Instruction for use | |
| Assay Type | Qualitative Detection |
| Specimen | Urine |
| Specification | Single panel 4.0mm |
| Certificates | ISO 13485,FDA 510K,Clia Waived,CE |
| Accuracy | Over 99% |
| Storage Temperature | 4-30 degree centigrade |
| Shelf Life | 2 years (24 months) |
| Delivery time | 15-30 days |
| OEM Services | Pouch, Box,Private Label, Insert |
| Loading Port | Guangzhou or Shenzhen, China |
Toxicology Urine test list
| Product Description | Abbreviations | Cut-off Level | Qualification | ||
| 1 | Amphetamine | AMP | 1000 ng/mL | CE | FDA 510K,CLIA-waived |
| 2 | Barbiturates | BAR | 300 ng/mL | CE | FDA 510K,CLIA-waived |
| 3 | Buprenorphine | BUP | 10 ng/mL | CE | FDA 510K,CLIA-waived |
| 4 | Benzodiazepines | BZO | 300 ng/mL | CE | FDA 510K,CLIA-waived |
| 5 | Cocaine | COC | 300 ng/mL | CE | FDA 510K,CLIA-waived |
| 6 | Cotinine | COT | 200 ng/mL | CE | |
| 7 | Ethylglucuronide | ETG | 500 ng/mL | CE | |
| 8 | Fentanyl | FYL | 200 ng/mL | CE | |
| 9 | Fentanyl | FYL | 1 ng/mL | FDA 510K | |
| 10 | Synthetic Cannabinoid | K2 | 50 ng/mL | CE | |
| 11 | Ketamine | KET | 1000 ng/mL | CE | |
| 12 | 6-Acetylmorphine | 6-MAM | 10 ng/mL | CE | |
| 13 | Methylenedioxymethamphetamine – ecstasy | MDMA | 500 ng/mL | CE | FDA 510K,CLIA-waived |
| 14 | Methamphetamine | MET | 1000 ng/mL | CE | FDA 510K,CLIA-waived |
| 15 | Morphine | MOP | 300 / 2000 ng/mL | CE | FDA 510K,CLIA-waived |
| 16 | Methadone | MTD | 300 ng/mL | CE | FDA 510K,CLIA-waived |
| 17 | Opiate 2000 | OPI | 2000 ng/mL | CE | |
| 18 | Oxycodone | OXY | 100 ng/mL | CE | FDA 510K,CLIA-waived |
| 19 | Phencyclidine | PCP | 25 ng/mL | CE | FDA 510K,CLIA-waived |
| 20 | Propoxyphene | PPX | 300 ng/mL | CE | FDA 510K,CLIA-waived |
| 21 | Tri-cyclic Antidepressants | TCA | 1000 ng/mL | CE | FDA 510K,CLIA-waived |
| 22 | Marijuana | THC | 50 ng/mL | CE | FDA 510K,CLIA-waived |
| 23 | Marijuana | THC | 20 / 50ng/mL | FDA 510K,CLIA-waived | |
| 24 | Tramadol | TRA | 200 ng/mL | CE | |
| 25 | Xylazine | XYL | 1000 ng/mL | ||
| 26 | Methaqualone | MQL | 300 ng/mL | CE | |
| 27 | Carisoprodol | SOMA | 1000 ng/mL | ||
| 28 | Methcathinone | MCAT | 500 ng/mL | ||
| 29 | Lysergic Acid Diethylamide | LSD | 20 ng/mL | ||
| 30 | Kratom | KRA | 500 ng/mL | ||
| 31 | 3,4-Methylenedioxypyrovalerone | MDPV | 1000 ng/mL | ||
| 32 | Tianeptine | TIA | 500 ng/mL | ||
| 33 | Gabapentin | GAB | 1000 ng/mL | ||
Optional Adulterant Strips: CR – Creatinine, OX – Oxidants, pH – Acidic or Alkaline, S.G – Specific Gravity, NIT – Nitrite, GLU – Glutaraldehyde
How to do the test?
Test must be at room temperature 59-86°F (15-30°C)
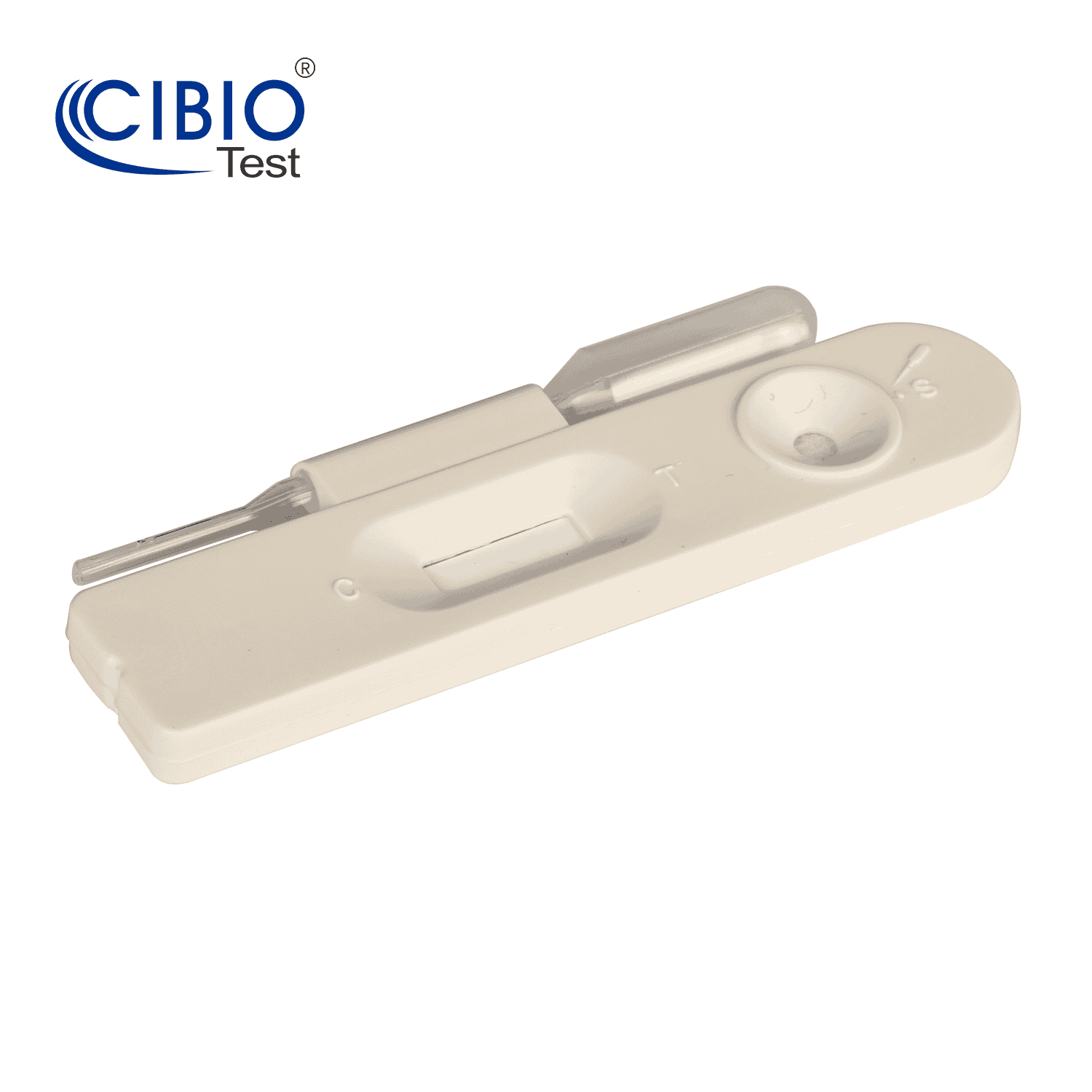
1.Remove a Testing Device from the foil pouch by tearing at the notch and place it on a level surface.
2. Holding a sample dropper vertically, add 3 drops of the urine specimen to the sample well (with an arrow marked).
3. Read the result in five minutes. Positive results may sometimes appear as early as one minute but you should wait the full 5 minutes before determining a negative result.
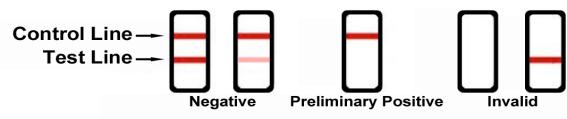
Reading the results
Preliminary positive (+)
A rose-pink band is visible in the control region, but no colored band appears in the test region. This indicates a preliminary positive result for this specific drug. Preliminary positive results must be sent to a lab for a more reliable test.
Negative (-)
A rose-pink band is visible in the control region and the test region. This indicates that the drug is not present in the sample or is at a level that cannot be detected by this test.
Invalid
If a color band is not visible in the control region, or a color band is only visible in the test region, the test is invalid. You should repeat the test with a new test dipcard. If the test still shows an invalid result, please contact us at 1-864-538-8007. When calling, be sure to provide the lot number for the test.
Note: There is no meaning to the colored line intensity or width. Any visible line is considered to be a line.
Q&A
Qus: How do rapid drug tests work?
Rapid drug tests detect the presence of drugs and drug metabolites in human urine at specified cut off levels in minutes. When a detection strip is wetted with urine specimen, the specimen reacts with the reagents and antigens on the strip and produces a color reading so you can interpret a positive or negative result.
Qus: What is the shelf life of your drug testing products?
The rapid drug test products have a shelf life of 24 months from manufacturing date. The expiration date is on every individual pack.
Qus: The drug line is lighter than the control line. Does this mean some drug is present?
No. Any line next to the word Drug or the drug abbreviation (depending on the test you have purchased), no matter how dark or light, is considered a Negative Result and no further testing is required.
Qus: Why is Urine Testing the Most Frequently Used Method for Drug Testing?
Businesses choose to drug test their workers and new job applicants to reduce the risk of workplace drug use and relevant hazards. While all drug test types, like hair follicle, blood, and saliva, have their benefits, urine drug testing is the most flexible and customizable.

-300x300.jpg)

Reviews
There are no reviews yet.